Enteral Feeding System Guide: Bags, ENFit Sets & Flush Syringes
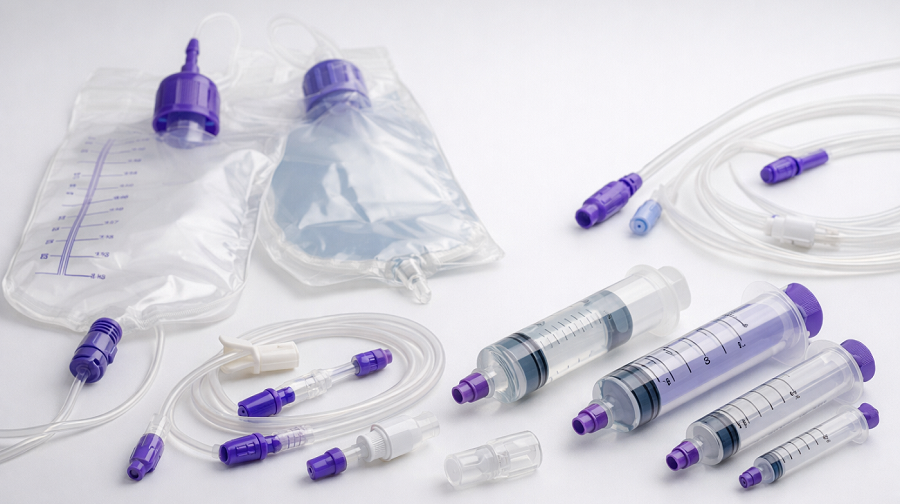
In enteral nutrition programs, the “small” consumables—feeding bags, connectors, and flush syringes—often determine whether care runs smoothly or turns into leakage, occlusions, and urgent tube replacements. As a manufacturer and supplier, we build enteral feeding systems for hospitals, distributors, homecare, and long-term care procurement teams who need consistent performance lot after lot.
This enteral feeding system guide focuses on three purchasing-critical elements: feeding bags, ENFit components, and flush syringes. I’ll share the practical checkpoints we use in production and QC so you can specify the right configuration, reduce user errors, and simplify purchasing across multiple care settings.
If you want to compare our standard configurations while you read, you can start from our medical disposable products page and drill into the enteral categories.
What “Good” Looks Like in an Enteral Feeding System
A complete enteral feeding system is not just a bag and a tube. In daily use, it must deliver predictable flow, maintain hygienic handling, and prevent connection errors—especially in environments where patients have multiple lines and devices.
Three outcomes procurement should target
- Stable delivery (gravity or pump) with reliable clamps and clear graduations to support measured dosing.
- Closed, leak-resistant connections that stay secure during patient movement, transfers, and medication administration.
- Misconnection-resistant interfaces—because wrong-route events are rare but high consequence.
When we help buyers standardize a system, we typically start by mapping the full workflow: fill → spike/prime → connect → deliver → flush → cap/secure → discard. The best specification is the one that matches that workflow with the least room for improvisation.
Feeding Bags: How to Specify the Right Bag for Gravity or Pump Use
Feeding bags do two jobs simultaneously: they are a reservoir and a metering aid. In procurement, the most common gap I see is specifying “1000 mL bag” without defining the delivery method, connector type, or handling features that prevent free-flow and contamination.
Key specifications that matter in practice
- Bag volume and graduations: common capacities run from 500 mL to 1200 mL. The graduation clarity and tolerance affect dosing confidence, especially for intermittent feeds.
- Tubing set length and kink resistance: long enough for bedside workflow, but not so long that it tangles during repositioning.
- Flow control: roller clamp quality and anti-free-flow behavior are more important than they look—small variations can change caregiver trust quickly.
- Material choice: buyers frequently request DEHP-free options and latex-free components to support facility policies.
| Decision point | Gravity feeding focus | Pump feeding focus |
|---|---|---|
| Flow control | Roller clamp precision and smooth adjustment | Pump compatibility and secure spike/port fit |
| User handling | Hang height variability; easy priming | Consistent set geometry for pump loading |
| Error prevention | Visible graduations and clear clamp status | Connector integrity to prevent micro-leaks during long runs |
Many facilities also standardize a replacement cadence to reduce contamination risk; a common operational target is single-patient, 24-hour use for feeding bags, especially in warm environments or high-touch workflows.
For typical bag-and-set configurations we manufacture, see our enteral feeding bag page.
ENFit Components: Reducing Wrong-Route Risk Without Slowing Care
ENFit components are designed to create an enteral-only connection pathway. From a buyer’s perspective, the goal is simple: connectors should be secure and intuitive for caregivers, and they should not be physically compatible with non-enteral ports.
Why this matters (with real-world safety context)
Wrong-route events are uncommon, but they are severe. In FDA reporting focused on enteral misconnections, the consequences included patient deaths and serious injuries. That’s exactly why many facilities standardize ENFit across feeding sets and syringes, rather than mixing connector ecosystems.
Procurement checklist for ENFit feeding sets
- Leak resistance under routine torque: connections should remain tight through normal handling and repositioning.
- Cap management and port hygiene: tethered caps and clear “open/closed” cues reduce contamination events.
- Transition support: if your facility still has legacy devices in circulation, specify adapters and a clear conversion plan to avoid improvised connections.
- Training consistency: packaging and labeling should make it difficult to grab the wrong set during busy shifts.
If you’re evaluating ENFit set options and typical component layouts, review our ENFit feeding sets page.
Flush Syringes: Preventing Occlusions and Improving Medication Delivery
Flush syringes are the “maintenance tool” of enteral therapy. When tubes clog, the impact is not just clinical—it becomes a logistics problem: replacement devices, extra nursing time, delayed feeding, and patient discomfort. A well-specified flush syringe reduces that risk and standardizes technique.
What to specify for enteral flushing workflows
- Volume selection: many protocols use larger syringes (for example, 30–60 mL) to deliver an effective flush without excessive pressure.
- Tip style and compatibility: specify the correct enteral connection interface (including ENFit where applicable) to prevent workarounds.
- Barrel clarity and graduations: clearer markings reduce “estimated flush” variability across caregivers.
- Sterile vs. non-sterile supply: match to the use case (acute care vs. homecare), and specify packaging format that supports your pick/pack flow.
A practical flushing example you can build into your SOP
- Flush before feeding to confirm patency and clear residual formula.
- Flush between medications to reduce incompatibilities and residue layering.
- Finish with a final flush and cap/secure the port to maintain hygiene.
For flush/irrigation-style syringes we supply (often used for tube flushing and general irrigation workflows), see our irrigation syringe page.
How the Components Fit Together: A Simple Compatibility Map
Buyers get the best results when they specify the system as a chain, not as isolated SKUs. A mismatch at any point—bag port to set, set to tube, tube to syringe—creates adapters, leakage risk, and training complexity.
| Component | Primary role | Common buyer checkpoint |
|---|---|---|
| Feeding bag | Reservoir + measured delivery support | Graduations, clamp reliability, material policy fit |
| ENFit feeding set | Secure enteral-only connection pathway | Leak resistance, cap hygiene, adapter strategy |
| Feeding tube | Patient interface for delivery | Kink resistance, markings, connector identification |
| Flush syringe | Maintain patency + administer flush/meds | Volume range, tip compatibility, packaging format |
If you’re standardizing the patient-interface portion of the system, you can also reference our feeding tube page to align tube markings and connector identification with your facility workflow.
Quality and Compliance: What I Recommend Buyers Audit
In our factory discussions with distributors and tenders, the strongest long-term relationships come from shared quality language. Instead of “good quality,” define measurable acceptance points that match how the product is actually used.
Incoming QC checks that catch the most real-world failures
- Leak testing on connectors and caps (focus on micro-leaks that appear only after twisting/torque).
- Clamp function sampling (smooth travel, no slippage, no sudden “jump” behavior).
- Graduation visibility under typical ward lighting (a frequent cause of dosing inconsistency).
- Packaging integrity and traceability (lot coding, clear labeling, and shelf-life control).
From a supplier qualification standpoint, many buyers also require documented QMS controls (for example, ISO 13485-aligned manufacturing) and consistent sterilization validation where sterile supply is requested. Those requirements reduce the probability of “surprise variability” between shipments—even when the SKU name stays the same.
OEM/ODM Options That Make Distribution Easier (Without Overcomplicating SKUs)
Customization is valuable when it reduces training time, pick errors, or total landed cost—not when it creates a catalog of near-duplicates. In our experience, the most useful OEM/ODM requests are the ones that standardize decision points for caregivers and logistics teams.
Common requests that improve usability and supply chain outcomes
- Private labeling and multilingual IFU/pack labeling to reduce rework in your warehouse.
- Kitting (bag + set + syringe) for homecare discharge packs or long-term-care monthly bundles.
- Standardizing connector ecosystems across product lines to minimize adapters and staff retraining.
- Packaging format optimization: single sterile vs. bulk non-sterile based on where the product is opened and used.
A simple example: if your distribution model serves both acute care and homecare, separating “sterile single-pack” from “bulk ward stock” while keeping the core geometry consistent can reduce SKU confusion while meeting each setting’s handling needs.
Buyer FAQs: Practical Questions We Answer During Qualification
Can I mix legacy and ENFit components during transition?
It’s possible, but I recommend treating it as a controlled transition, not a permanent mixed environment. Define where adapters are permitted, how they’re stored, and how you’ll prevent “creative” rigging that defeats safety goals.
What should I standardize first: bags, sets, or syringes?
Start with the connection points that create the most downstream complexity. In many tenders, standardizing the connector ecosystem (ENFit pathway) first reduces wrong-item selection and adapter creep across the entire enteral program.
What’s the simplest way to reduce field complaints?
Specify measurable acceptance criteria (leak checks, clamp performance sampling, graduation visibility) and require traceability that supports fast root-cause analysis. The fastest supplier response happens when we can isolate a lot, review production records, and validate corrective action quickly.
If you’d like us to recommend a standardized “system spec” for your market (hospital tender, distributor catalog, or homecare kit), we typically start from your target use setting, connector policy (ENFit/transition), and packaging preference—and then align bag, set, and flush syringe options around that.
Bottom line: a well-matched set of feeding bags, ENFit components, and flush syringes reduces workflow friction, lowers avoidable tube occlusions, and makes staff training and purchasing far more predictable.

 English
English Français
Français Español
Español Português
Português عربى
عربى