Product Quality & Technical Specifications
What specific mesh counts (e.g., 20x12, 26x18) are available, and how do they impact absorbency and cost?
We offer an extremely wide selection of mesh counts, including but not limited to low density counts such as 7(12x8) and 13(19x15); medium-high density counts such as 21(28x24); and high density counts such as 38(50x48). Higher mesh counts generally result in greater softness and faster absorbency but also come with a correspondingly higher cost. We accept customization options in this regard.
How do you guarantee the absorbency rate and fluid retention capacity meet international standards (e.g., USP/BP)?
Our gauze is made from 100% pure cotton, ensuring high absorbency. Through our internal Quality Control (QC) process, we regularly conduct absorbency tests (such as immersion time tests). Our products comply with the absorption performance requirements of the United States Pharmacopeia (USP) and the British Pharmacopoeia (BP).
Is the gauze material 100% pure cotton, and what is your process for ensuring material purity and safety?
Yes, our gauze products are made from 100% pure cotton. We conduct rigorous audits of our raw material suppliers and are committed to testing materials for harmful residues such as fluorescent agents and heavy metals.
What sterilization methods are used for sterile gauze, and what is the guaranteed sterility assurance level (SAL)?
We provide products in sterile packaging. Sterilization methods typically include Ethylene Oxide (EO) or Gamma irradiation. The standard guaranteed Sterility Assurance Level (SAL) is 10-6.
Procurement Cost & Value
What is your tiered pricing structure for high-volume, recurring orders?
We offer a quantity-based tiered discount model. Please provide your estimated procurement volume, and we will provide the corresponding Minimum Order Quantity (MOQ) and price levels. Long-term cooperation clients enjoy additional preferential terms.
Beyond unit price, what is your product's total value proposition (e.g., reduced waste, better clinical performance)?
Our value proposition lies in providing stable, high-quality products that reduce the frequency of changes and overall usage, thereby lowering the hospital's Total Cost of Ownership (TCO).
What are the available Incoterms (e.g., FOB, CIF) for global shipping, and how are logistics costs calculated?
We support major International Commercial Terms (Incoterms), such as FOB and CIF. Logistics costs are calculated based on order volume, destination, and the chosen method of transport (sea/air freight), and we provide transparent quotations.
Supplier Credibility & Reliability
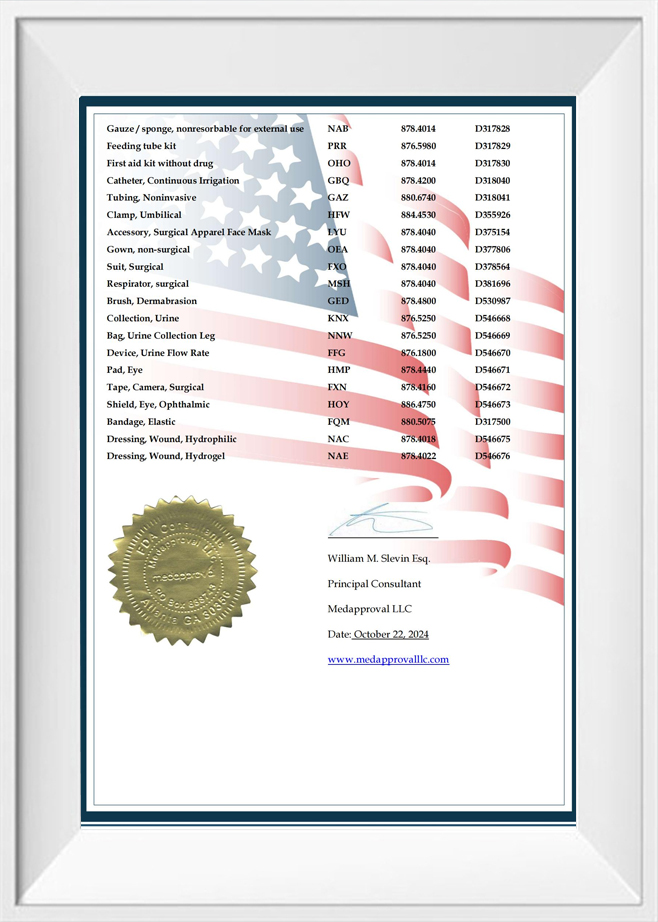
Can you provide evidence of regulatory compliance (e.g., ISO 13485, CE Mark, FDA registration) for the target markets?
We hold the CE Mark and ISO 13485 certifications issued by TUV and are registered with the FDA. These certifications cover our main product lines.
What is your typical lead time for a large-scale order (e.g., 1 million units), and what is your production capacity?
Our factory possesses strong monthly production capacity, enabling us to meet bulk order requirements. Please contact us for an assessment of the specific lead time.
Can we visit your manufacturing facility for an audit, and do you provide references from existing global clients?
Clients are welcome to conduct factory audits; please provide advance notice to arrange the process. We can provide relevant success stories or references upon obtaining permission from existing clients.
Packaging and Specification Variety
What is your range of standard sizes (e.g., 2x2, 4x4, 4x8) and ply options, and can you accommodate custom sizes?
We offer a variety of standard sizes and ply options (e.g., 8-ply, 12-ply). Gauze roll widths include 36”, 100cm, etc.; gauze ball diameters include 1.5cm, 2cm, etc. We accept custom sizes, subject to meeting the Minimum Order Quantity (MOQ).
How is product traceability ensured throughout the supply chain, from manufacturing to delivery?
We employ a strict batch control system. Every package features a clear batch number, production date, and expiration date, allowing for rapid traceability back to raw materials and production records in the event of a quality issue.
Do you offer different packaging formats (e.g., sterile unit packs, bulk non-sterile bags, dispenser boxes)?
Yes, we offer multiple packaging formats. For example, gauze balls are available in sterile unit packs (e.g., 1pc/pack, 2pcs/pack) and non-sterile bulk packaging (e.g., 100pcs/pack).

 English
English Français
Français Español
Español Português
Português عربى
عربى